Tokyo, 25 December, /AJMEDIA/
Earth is full of natural magnesium, forged long ago in the stars, that has since become a key component of our diets and minerals in the planet’s crust.
But this magnesium is stable. Its atomic core, or nucleus, doesn’t fall apart.
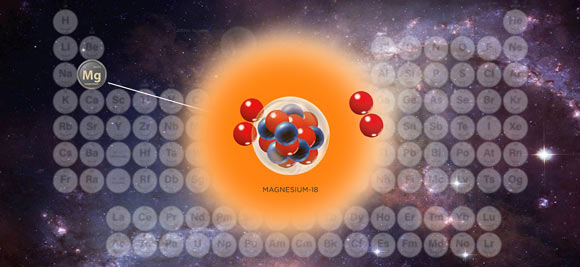
The magnesium-18 isotope, however, is far too unstable to be found in nature.
All magnesium atoms have 12 protons inside their nuclei. Previously, the lightest version of magnesium had 7 neutrons, giving it a total of 19 protons and neutrons — hence its designation as magnesium-19.
To make magnesium-18, which is lighter by one neutron, Dr. Kyle Brown from the National Superconducting Cyclotron Laboratory at Michigan State University and colleagues started with a stable isotope of magnesium, magnesium-24.
The cyclotron at the National Superconducting Cyclotron Laboratory accelerated a beam of magnesium-24 nuclei to about half the speed of light and sent that beam barreling into a target, which is a metal foil made from the element beryllium. And that was just the first step.
“That collision gives you a bunch of different isotopes lighter than magnesium-24,” Dr. Brown said.
“But from that soup, we can select out the isotope we want.”
In this case, that isotope is magnesium-20. This version is unstable, meaning it decays, usually within tenths of a second.
So the team is on a clock to get that magnesium-20 to collide with another beryllium target about 30 m (100 feet) away.
“But it’s traveling at half the speed of light. It gets there pretty quickly,” Dr. Brown said.
It’s that next collision that creates magnesium-18, which has a lifetime somewhere in the ballpark of a sextillionth of a second.
That’s such a short time that magnesium-18 doesn’t cloak itself with electrons to become a full-fledged atom before falling apart. It exists only as a naked nucleus.
In fact, it’s such a short time that magnesium-18 never leaves the beryllium target. The new isotope decays inside the target.
This means scientists can’t examine the isotope directly, but they can characterize tell-tale signs of its decay.
It first ejects two protons from its nucleus to become neon-16, which then ejects two more protons to become oxygen-14.
By analyzing the protons and oxygen that do escape the target, the team can deduce properties of magnesium-18.
“This was a team effort. Everyone worked really hard on this project. It’s pretty exciting. It’s not every day people discover a new isotope,” Dr. Brown said.
The results were published in the journal Physical Review Letters.