Tokyo, 2 March, /AJMEDIA
In November, a new coronavirus variant took the world by storm. Omicron has since caused an unprecedented wave of infections, striking about 90 million people in just 10 weeks. That’s more COVID-19 cases than were recorded in all of 2020.
Omicron also left scientists scratching their heads. It’s riddled with mutations, which might normally doom a virus. Early experiments showed that omicron wasn’t nearly as good as the previous coronavirus variant champ, delta, at melding with a cell’s membrane — crucial for infecting that cell — or at replicating in lung cells. Yet here it was, sweeping delta virtually off the map in just weeks in some places (SN: 2/10/22). Omicron even managed to infect people who already had immunity to the virus from vaccines or previous cases of COVID-19. How, researchers wondered, was omicron doing it?
“It’s a very interesting variant,” says virologist Shan-Lu Liu, who codirects the Viruses and Emerging Pathogens Program at The Ohio State University in Columbus. “I call it weird.”
Researchers in Botswana and South Africa were the first to unveil omicron’s genetic makeup. Their analysis revealed more than 60 mutations, including 42 changes alone in omicron’s spike protein — the knobby structure on the surface of the virus that initiates a cell break-in and can help evade antibody defenses. Some of those mutations have popped up in previous variants, including alpha and delta. But omicron has never-before-seen tweaks and unique combinations of mutations.
Scientists have been scrambling to discover how those changes affect omicron’s ability to infect people and cause disease. Researchers around the world are infecting cells in lab dishes with omicron mimics, putting the virus under the microscope, testing the viral variant in lab animals and examining medical and other records all to discover what makes the variant tick. Here’s what scientists have found so far.
1. Omicron keeps it together.
The secret of omicron’s increased transmissibility has proven elusive. The spike protein is certainly one key to its success, allowing omicron to infect nearly 10 times as many cells as earlier versions of the virus, researchers in China reported December 17 in Signal Transduction and Targeted Therapy.
It is “distinct from all other variants, from a structural point of view,” says structural biologist Priyamvada Acharya. She and her colleagues at the Duke Human Vaccine Institute in Durham, N.C., used cryo-electron microscopy and other techniques to examine omicron’s spike protein.
In past studies, researchers, including Acharya, have pinpointed how certain mutations have given previous variants a leg up. For instance, some mutations in the delta variant’s spike protein helped that version of the coronavirus more easily grab a human protein called ACE2 or more readily fuse with human cells (SN: 12/16/21). Omicron shares some of those mutations but has many others that drastically change how the variant behaves. “It’s not just one thing,” Acharya says. “It’s multiple properties of the spike that confer an advantage to omicron.”
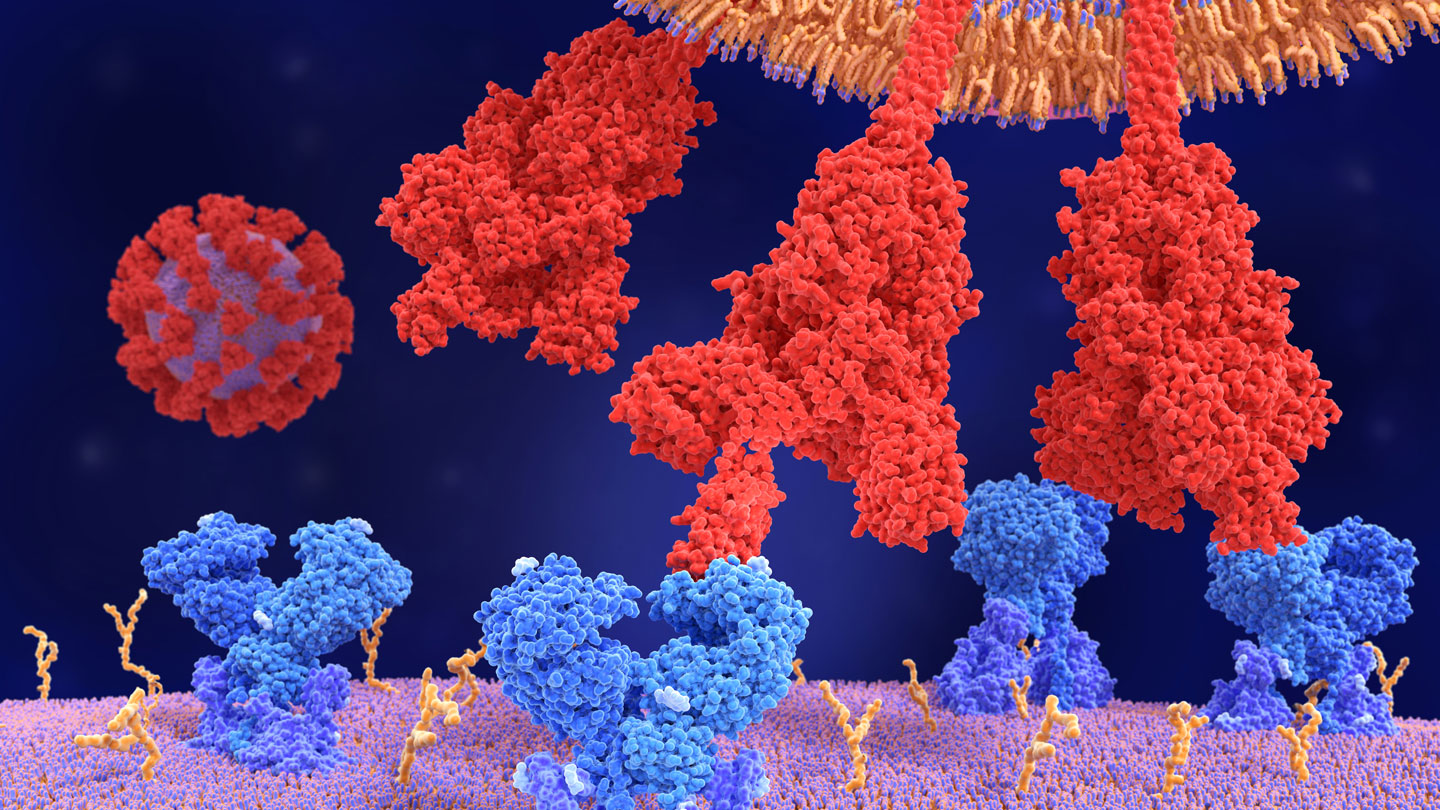
Like other versions of the coronavirus, each of omicron’s knobby spikes consists of three identical pieces that snap together in a single unit. Each of those pieces has a jointed, fingerlike portion called the receptor binding domain that reaches out, much like the prongs of a claw machine, to grasp ACE2 and anchor the virus to the cell it will infect.
Many antibodies that prevent the virus from entering cells target those fingers. But omicron keeps its knuckles bent, hiding the bits that antibodies will attack, Acharya and colleagues reported in a preprint, which has not been peer reviewed yet, posted January 26 at bioRxiv.org.
This closed-fist strategy helps the variant evade the immune system. But the fingers eventually have to extend in order to grasp ACE2. Some mutations essentially spring-load omicron’s claw so that it can shoot out at least one finger to snag ACE2. That ensures some of the spike proteins studding the surface of the virus are always pointing the way to infect cells, Acharya and colleagues found.
Delta and alpha variants of the coronavirus were also likely to have multiple extended fingers, but those variants’ efforts to open up “went way too far … to the point that they lost control and fell apart,” Acharya says. Omicron contains mutations that stabilize the spike protein through hydrogen bonds — weak electrostatic connections between a positively charged hydrogen atom in one molecule with a negatively charged atom in another molecule. As a result, omicron doesn’t become floppy the way the earlier variants do, the Duke group discovered. Another group also found new hydrogen bonds and other connections that help omicron keep itself together, researchers reported January 20 in Science. “I think omicron has struck just the right balance,” Acharya says.
2. With omicron, opposites really attract.
Simple attraction may be one key to omicron’s success.
“In the cellular environment, it can be difficult to find your partner,” says computational biologist Hin Hark Gan of New York University. Mutations in omicron’s receptor binding domain give that fingerlike portion of the protein a positive electric charge, Gan and NYU colleagues in New York City and Abu Dhabi reported February 14 at bioRxiv.org.
That electric charge complements ACE2’s negative charge, creating an electrostatic force that attracts the two proteins like a static-charged balloon to a wall, even over relatively long distances, the researchers propose in their preliminary report. Omicron’s electrostatic attraction to ACE2 is three to five times greater than more neutrally charged delta’s, the team found. The electrical attraction may make it easier for omicron to locate ACE2 on cells. Once the virus gets close to the human protein, other types of forces, including hydrogen bonds, cement the connection, he says.
3. Omicron may sneak in a cell’s back door.
Multiple teams around the world have recently proposed another thing that makes omicron special: The variant may not use the same entry route into cells that earlier versions of the virus use.
There are two major ways the coronavirus can enter cells (SN: 8/2/20). Both start with grabbing ACE2. In the direct route, a scissorslike protein called TMPRSS2 snips away part of the spike protein, revealing a portion that allows the virus to fuse with human cells and immediately dump its RNA inside to make new viruses. That is the way all previous versions of the SARS-CoV-2 have entered human cells.
But omicron may take a back door through a compartment inside the cell membrane called an endosome. There, a different scissorslike protein called cathepsin L cleaves the spike protein to allow the virus to dump its payload into the cell.
Omicron doesn’t use the TMPRSS2 pathway efficiently and relies more on cathepsin L to get into cells, two groups of researchers independently reported February 1 in Nature. As a result, omicron doesn’t fuse as well with cell membranes as delta does, those teams and other preliminary reports suggest.
That could seem like a handicap. But for omicron, it may be a good thing, Liu of Ohio State says. While the virus needs to fuse with cell membranes to get inside and replicate, too much fusibility may lead cells to merge with each other and die, he says. That would leave the virus with nowhere to copy itself.
Omicron may have struck the perfect balance between being fusible enough to enter cells, but not enough to kill its host, he says. This characteristic may also make omicron less likely to cause severe disease.